Time Reduction
Documents Automated
Happy Clients
Efficiency Increase
Comprehensive compliance solutions designed for the pharmaceutical and manufacturing industries
Automates compliance documents, speeding pharma and manufacturing workflows.
Pre-built templates ensure effortless compliance with GMP, ISO, FDA standards.
We streamline document lifecycle, enabling scalable, multi-industry enterprise operations.
Comprehensive solutions to transform your compliance processes
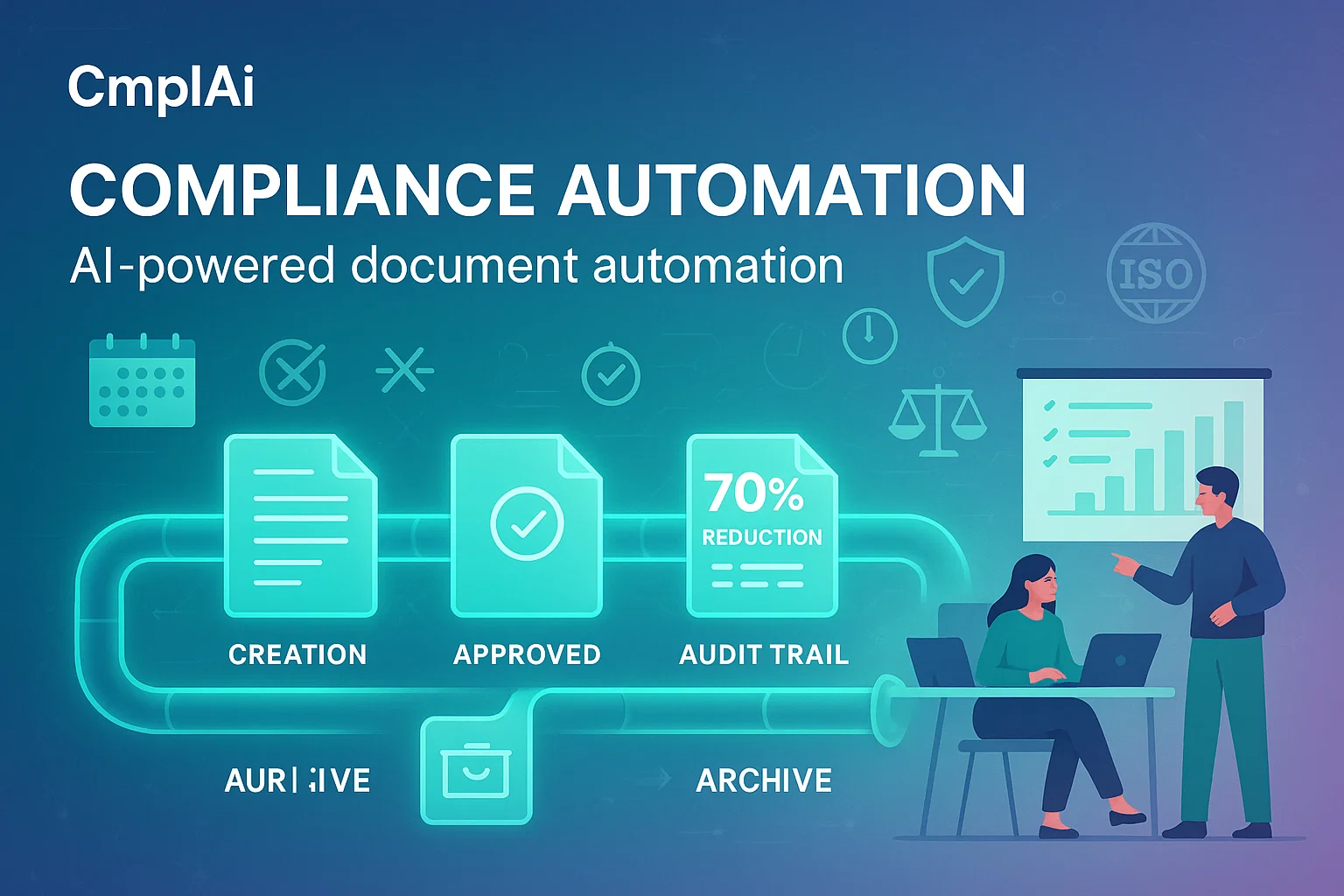
AI-powered document automation reducing compliance workload by 70%
Regulatory-ready templates ensuring 100% compliance with global standards
End-to-end document lifecycle management with full audit trails
Reduced turnaround time from months to days
Minimized manual errors and enhanced data integrity
Improved resource allocation and team productivity
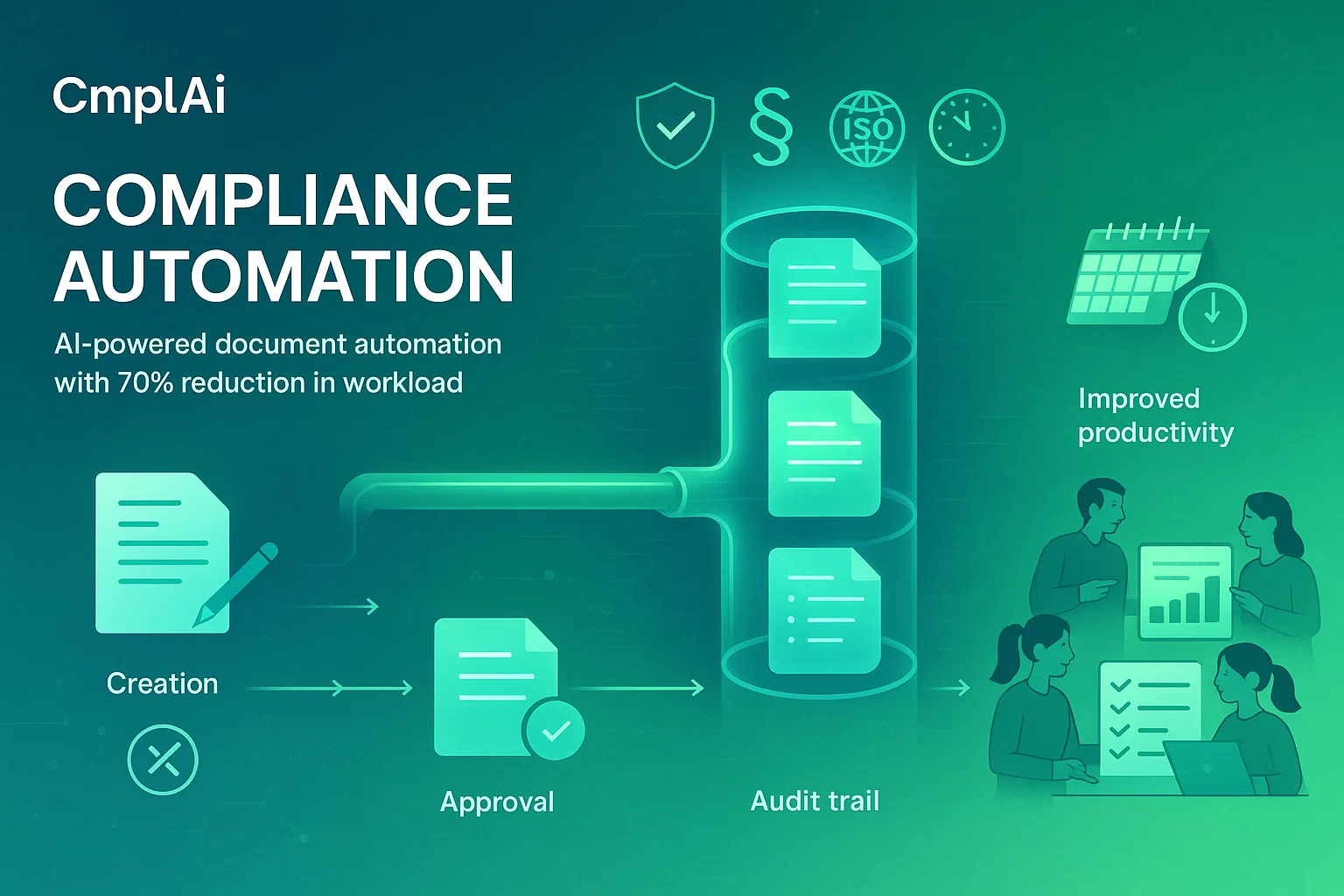
Discover how Cmplai transforms compliance processes
Comply revolutionizes compliance by automating document preparation, reducing turnaround time for critical workflows from 3 months to just 3 days. Our GenAI-powered SaaS ERP platform minimizes manual intervention and errors, ensuring unmatched accuracy and regulatory adherence.
We deliver pre-built templates and compliance logic tailored to GMP, ISO, US-FDA, and other global standards, so your documentation is always audit-ready and meets the strictest requirements.
We reduce repetitive manual tasks, allowing your teams to focus on high-value work like innovation and quality enhancement, leading to better resource allocation and job satisfaction.
Common compliance pain points faced by pharmaceutical and manufacturing companies
Preparing, reviewing, and managing thousands of compliance documents manually leads to significant errors, inefficiencies, and compliance risks, with each plant spending over 100,000 hours annually on documentation.
Keeping up with rapidly changing global regulatory requirements (GMP, ISO, US-FDA, etc.) is difficult, often resulting in delays and increased risk of non-compliance.
Maintaining complete, accurate, and up-to-date documentation for audits is challenging, especially with legacy systems and manual data entry that threaten data integrity.
Key milestones in our growth story
LN Infosphere TechTransformers Pvt Ltd officially registered
Incubated at iTIC IIT Hyderabad
Incubated at IITM Incubation Cell
Received grant of 4 lakhs from iTIC
Our mission and vision

At Cmplai, we're on a mission to revolutionize how pharmaceutical and manufacturing companies handle compliance documentation. Founded by industry experts with decades of experience, we understand the challenges organizations face with regulatory compliance.
Our team combines deep domain expertise in pharmaceutical compliance with cutting-edge AI technology to create solutions that dramatically reduce the time, cost, and risk associated with compliance documentation.
We believe that by automating the most tedious aspects of compliance, we can free up human talent to focus on innovation and quality improvement — ultimately leading to better products and services for consumers worldwide.